Awardees

Deborah Bruner, SVP Research; Catherine Murari-Kanti, case manager; Priscilla Delgado, inventor; Todd Sherer, AVP Research; David Myers and Nicholas Au Yong, inventors
Innovation of the Year: A Bio-Inspired Skin Interface Method for Continuous Access to Blood for Measurement and Therapy; David Myers, PhD; Nicholas Au Yong, PhD; Priscilla Delgado
On average, it takes at least 2-3 hours for a physician to receive results from a blood test. Measurements of blood are typically performed by drawing a patient’s blood, sending the blood to a testing lab, with the results being reported to the physician. This delay may sometimes mean life or death depending on how the results affect treatment outcomes. In many instances, quicker results would allow for real-time treatment. There is a need to continually collect blood and measure its components, but the largest challenge to do so is blood clotting. However, systematic clotting inhibitors pose a great risk to the patient. To address this concern, David Myers, PhD, Nicholas Au Yong, PhD; and Priscilla Delgado have developed a wearable, continuous blood collection device. The device will be a patch that allows for continuous collection of blood, preventing blood clotting as well as monitoring of blood-based biomarkers using sensors embedded in the device.

Deborah Bruner, SVP Research; Raj Guddneppanavar, case manager; Eric Ortlund, inventor; Todd Sherer, AVP Research
Deal of the Year: Allonix Therapeutics – Liver Receptor Homolog-1 (LRH-1) Modulators as Potential Therapeutics for Metabolic, Cancer, and Inflammatory Diseases; Eric Ortlund, PhD
Human nuclear receptors are a superfamily of ligand-responsive transcription factors that have central roles in cellular function. The ability of nuclear receptors to control gene programs in response to small lipophilic molecules makes them ideal pharmaceutical targets for a wide range of metabolic and neoplastic diseases, with 10–20% of FDA-approved medications currently targeting nuclear receptors. Liver receptor homolog-1 (LRH-1) is a nuclear hormone receptor known to control diverse transcriptional programs in different tissues related to metabolism, inflammation, and cellular proliferation. Discovery of LRH-1 modulators has been difficult, in part due to the tendency for synthetic compounds to bind unpredictably within the lipophilic binding pocket. Dr. Eric Ortlund and his research team using a structure-guided approach, exploited a newly discovered polar interaction to lock agonists in a consistent orientation. This enabled the discovery of the first low nanomolar LRH-1 agonist, one hundred times more potent than the best previous modulator. Since then, the Emory team has developed LRH-1 modulators as potential therapeutics for metabolic, cancer and inflammatory diseases. In 2023, Emory University executed an exclusive high net worth license with Allonix Therapeutics for this technology.

Patrick Reynolds, case manager; Deborah Bruner, SVP Research; Ýmir Vigfússon, inventor; Todd Sherer, AVP Research
Start-Up of the Year: Ýmir Vigfússon, PhD
The last 30 years of computer development and cybersecurity have had one constant: it’s easy to hack a computer. The level of sophistication of modern attack tools makes detecting whether a computer has been hacked incredibly difficult. Modern cybersecurity is unable to detect if an end-user is physically sitting at an authorized device, or if it has been compromised by a hacker seeking to gain further access to a remote server. To help address this issue, Ýmir Vigfússon, PhD developed “KeyStrike”, a small USB device that connects to the USB port of a computer. When an end-user enters a key on their keyboard, KeyStrike will forward the keystroke with additional keystrokes. If the server receives a keystroke from the user without the corresponding additional characters, it can be known immediately that the computer may be compromised. Without access to the secret key, the hack would be detected with overwhelming probability. Since KeyStrike was awarded Innovation of the Year at the 2022 Annual Celebration, they haven’t slowed down. They were accepted into and participated in the Berkeley Skydeck program, licensed their IP from OTT, raised $1.3M, obtained customers, and grew the company to 14 employees.

Deborah Bruner, SVP Research; Mark Coburn, case manager; Daryll Vanover accepting for Philip Santangelo, inventor; Todd Sherer, AVP Research
Significant Event of the Year: ARPA-H Funding; Philip Santangelo, PhD
In 2023, Emory University was selected as the inaugural recipient of funding from the Advanced Research Projects Agency for Health (ARPA-H), a new federal agency within the U.S. Department of Health & Human Services. This transformative three-year, $24.8 million cooperative agreement aims to drive groundbreaking health research and catalyze transformative breakthroughs in the field. The focus of ARPA-H was to identify innovative approaches that could prevent, treat, and potentially cure diseases such as cancer, autoimmune disorders, and infectious diseases. These conditions often arise due to dysregulation of the immune system, impairing the body’s ability to control immune responses and leaving patients vulnerable. Dr. Philip Santangelo, a distinguished professor in the Wallace H. Coulter Department of Biomedical Engineering at Emory University and the Georgia Institute of Technology, emerged as a key player in this endeavor. His groundbreaking work centers around a novel class of mRNA-based drugs designed to precisely “turn on or turn off” genes in individual immune cells. By combining mRNA-encoded antigens with gene modulation technology, Dr. Santangelo’s approach aims to radically enhance specific immune responses. In summary, Dr. Santangelo’s pioneering work, supported by ARPA-H, holds the promise of transforming immune modulation and advancing our ability to combat challenging diseases. The collaboration between Emory University and its research partners exemplifies a commitment to innovation and impactful health outcomes.

Janette Hannam-Hayes, OSP; Deborah Bruner, SVP Research; Todd Sherer, AVP Research; Erica Evans, Kirby Fibben, Wilbur Lam, Vivien Sheehan inventors
Corporate Partnership of the Year: Honeywell; Wilbur Lam, MD, PhD; Vivien Sheehan, MD, PhD; Christina Caruso, MD; Evelyn Kendall Williams, PhD; Erica Evans; Kirby Fibben
Honeywell, a global leader in innovation across multiple industries, had an idea for a COVID-19 diagnostic and needed access to clinical and scientific expertise. At first, they approached the NIH, who recommended the company to Emory. Upon agreement to collaborate with Emory, Honeywell and Wilbur Lam, MD, PhD, and his team began work on the diagnostic. Ultimately, Honeywell pulled the program, but they were still interested in working with Emory. Intrigued by Lam’s and Vivien Sheehan, MD, PhD’s work on sickle cell disease (SCD), Honeywell began anew with a multi-pronged, multi-year partnership to develop multiple diagnostics for SCD. As of now, the team – including not only Lam, Sheehan, and their labs, but also Evelyn Williams, PhD; Christina Caruso, MD; Erica Evans; and Kirby Fibben – is co-developing with Honeywell and jointly filing patents. As the relationship continues, more patents will be filed for their technologies, as Dr. Lam and Honeywell see the potential to develop diagnostics for infectious and cardiovascular diseases in the years to come.
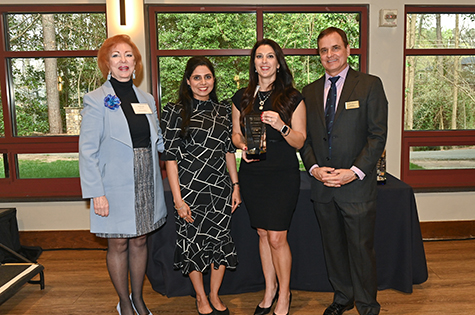
Deborah Bruner, SVP Research; Shweta Ghai, case manager; Louise Hecker, inventor; Todd Sherer, AVP Research
EmpowHER Award: Louise Hecker, PhD
Dr. Louise Hecker is a prominent researcher in the field of regenerative biology, with a specialty in pulmonology. Her research primarily focuses on understanding the molecular mechanisms underlying lung fibrosis and developing novel therapeutic approaches for treating this debilitating disease. Throughout her career, Dr. Hecker has made significant contributions to the field, particularly in identifying the role of Nox4 (NADPH oxidase-4) in creating scar tissue in the lungs. Dr. Hecker’s goal is to stop Nox4 by developing improved therapies that would help those affected by fibrotic disorders lead longer, healthier lives. Dr. Hecker has received numerous awards and honors for her work, including grants from the National Institutes of Health (NIH) and the U.S. Departments of Defense and Veterans Affairs. She was also named a Distinguished Investigator by the Georgia Research Alliance (GRA) upon joining Emory. In addition, Dr. Hecker has founded two start-up companies related to her research. She has published extensively in peer-reviewed journals and continues to lead innovative research projects aimed at advancing our understanding of pulmonary fibrosis and developing more effective treatments for patients suffering from this condition.

Deborah Bruner, SVP Research; Meichen Wei, Christina Calamaro, inventors; Sat Balachander, case manager; Santiago Arconada Alvarez, Wilbur Lam, Michael Fundora, inventors; Todd Sherer, AVP Research
Inclusivity, Diversity, and Equity Award (IDEAward): Low English Proficiency Nurse Communication Tool; Christina Calamaro, PhD; Michael Fundora, MD; Morgan Greenleaf; Santiago Arconada Alvarez; Wilbur Lam, MD, PhD
Although the United States continues to become more diverse, English remains the primary language spoken. For patients with limited language proficiency (LLP), navigating a healthcare system that is predominantly English-speaking can be overwhelming. Today, over 8% of the U.S. population identifies as LLP. A language barrier leads to a greater potential for medical errors, therefore a solution to increase the quality and equity of healthcare for LLP patients is urgently needed. Limited language proficiency patients report lower satisfaction overall with health encounters, and they are less likely to understand medical diagnoses and treatment plans. The inventors propose a novel mobile application that enables the clinical team to communicate using common phrases in the patient’s language, images, as well as real-time translations. The app, designed with a pediatric setting in mind, encourages rapid and accurate communication with families by eliminating the wait for an interpreter to be available. Drs. Calamaro and Fundora developed the app in partnership with the Emory AppHatchery. In the future,the team hopes to expand the app’s use to adult patients.

Catherine Murari-Kanti, case manager; Phillip Zhe Sun, inventor; Robert Nobles, VP Research Admin
Innovation of the Year: A Bio-Inspired Skin Interface Method for Continuous Access to Blood for Measurement and Therapy; David Myers, PhD; Nicholas Au Yong, PhD; Priscilla Delgado
On average, it takes at least 2-3 hours for a physician to receive results from a blood test. Measurements of blood are typically performed by drawing a patient’s blood, sending the blood to a testing lab, with the results being reported to the physician. This delay may sometimes mean life or death depending on how the results affect treatment outcomes. In many instances, quicker results would allow for real-time treatment. There is a need to continually collect blood and measure its components, but the largest challenge to do so is blood clotting. However, systematic clotting inhibitors pose a great risk to the patient. To address this concern, David Myers, PhD, Nicholas Au Yong, PhD; and Priscilla Delgado have developed a wearable, continuous blood collection device. The device will be a patch that allows for continuous collection of blood, preventing blood clotting as well as monitoring of blood-based biomarkers using sensors embedded in the device.

Zhe Chen, Raymond Schinazi, HongWang Zhang, Mahesh Kasthuri, Frank Amblard, Longhu Zhou, inventors; Robert Nobles, VP Research Admin
Deal of the Year: Allonix Therapeutics – Liver Receptor Homolog-1 (LRH-1) Modulators as Potential Therapeutics for Metabolic, Cancer, and Inflammatory Diseases; Eric Ortlund, PhD
Human nuclear receptors are a superfamily of ligand-responsive transcription factors that have central roles in cellular function. The ability of nuclear receptors to control gene programs in response to small lipophilic molecules makes them ideal pharmaceutical targets for a wide range of metabolic and neoplastic diseases, with 10–20% of FDA-approved medications currently targeting nuclear receptors. Liver receptor homolog-1 (LRH-1) is a nuclear hormone receptor known to control diverse transcriptional programs in different tissues related to metabolism, inflammation, and cellular proliferation. Discovery of LRH-1 modulators has been difficult, in part due to the tendency for synthetic compounds to bind unpredictably within the lipophilic binding pocket. Dr. Eric Ortlund and his research team using a structure-guided approach, exploited a newly discovered polar interaction to lock agonists in a consistent orientation. This enabled the discovery of the first low nanomolar LRH-1 agonist, one hundred times more potent than the best previous modulator. Since then, the Emory team has developed LRH-1 modulators as potential therapeutics for metabolic, cancer and inflammatory diseases. In 2023, Emory University executed an exclusive high net worth license with Allonix Therapeutics for this technology.

Patrick Reynolds, case manager; David Prologo, inventor; Robert Nobles, VP Research Admin
Start-Up of the Year: Ýmir Vigfússon, PhD
The last 30 years of computer development and cybersecurity have had one constant: it’s easy to hack a computer. The level of sophistication of modern attack tools makes detecting whether a computer has been hacked incredibly difficult. Modern cybersecurity is unable to detect if an end-user is physically sitting at an authorized device, or if it has been compromised by a hacker seeking to gain further access to a remote server. To help address this issue, Ýmir Vigfússon, PhD developed “KeyStrike”, a small USB device that connects to the USB port of a computer. When an end-user enters a key on their keyboard, KeyStrike will forward the keystroke with additional keystrokes. If the server receives a keystroke from the user without the corresponding additional characters, it can be known immediately that the computer may be compromised. Without access to the secret key, the hack would be detected with overwhelming probability. Since KeyStrike was awarded Innovation of the Year at the 2022 Annual Celebration, they haven’t slowed down. They were accepted into and participated in the Berkeley Skydeck program, licensed their IP from OTT, raised $1.3M, obtained customers, and grew the company to 14 employees.

Mark Coburn, case manager; Murali Padala, inventor; Robert Nobles, VP Research Admin
Significant Event of the Year: ARPA-H Funding; Philip Santangelo, PhD
In 2023, Emory University was selected as the inaugural recipient of funding from the Advanced Research Projects Agency for Health (ARPA-H), a new federal agency within the U.S. Department of Health & Human Services. This transformative three-year, $24.8 million cooperative agreement aims to drive groundbreaking health research and catalyze transformative breakthroughs in the field. The focus of ARPA-H was to identify innovative approaches that could prevent, treat, and potentially cure diseases such as cancer, autoimmune disorders, and infectious diseases. These conditions often arise due to dysregulation of the immune system, impairing the body’s ability to control immune responses and leaving patients vulnerable. Dr. Philip Santangelo, a distinguished professor in the Wallace H. Coulter Department of Biomedical Engineering at Emory University and the Georgia Institute of Technology, emerged as a key player in this endeavor. His groundbreaking work centers around a novel class of mRNA-based drugs designed to precisely “turn on or turn off” genes in individual immune cells. By combining mRNA-encoded antigens with gene modulation technology, Dr. Santangelo’s approach aims to radically enhance specific immune responses. In summary, Dr. Santangelo’s pioneering work, supported by ARPA-H, holds the promise of transforming immune modulation and advancing our ability to combat challenging diseases. The collaboration between Emory University and its research partners exemplifies a commitment to innovation and impactful health outcomes.

Janette Hannam-Hayes, OSP; Deborah Bruner, SVP Research; Anant Madabhushi, inventor; Robert Nobles, VP Research Admin; Sat Balachander, case manager
Corporate Partnership of the Year: Honeywell; Wilbur Lam, MD, PhD; Vivien Sheehan, MD, PhD; Christina Caruso, MD; Evelyn Kendall Williams, PhD; Erica Evans; Kirby Fibben
Honeywell, a global leader in innovation across multiple industries, had an idea for a COVID-19 diagnostic and needed access to clinical and scientific expertise. At first, they approached the NIH, who recommended the company to Emory. Upon agreement to collaborate with Emory, Honeywell and Wilbur Lam, MD, PhD, and his team began work on the diagnostic. Ultimately, Honeywell pulled the program, but they were still interested in working with Emory. Intrigued by Lam’s and Vivien Sheehan, MD, PhD’s work on sickle cell disease (SCD), Honeywell began anew with a multi-pronged, multi-year partnership to develop multiple diagnostics for SCD. As of now, the team – including not only Lam, Sheehan, and their labs, but also Evelyn Williams, PhD; Christina Caruso, MD; Erica Evans; and Kirby Fibben – is co-developing with Honeywell and jointly filing patents. As the relationship continues, more patents will be filed for their technologies, as Dr. Lam and Honeywell see the potential to develop diagnostics for infectious and cardiovascular diseases in the years to come.

Sat Balachander, case manager; Cassandra Quave, inventor; Robert Nobles, VP Research Admin
EmpowHER Award: Louise Hecker, PhD
Dr. Louise Hecker is a prominent researcher in the field of regenerative biology, with a specialty in pulmonology. Her research primarily focuses on understanding the molecular mechanisms underlying lung fibrosis and developing novel therapeutic approaches for treating this debilitating disease. Throughout her career, Dr. Hecker has made significant contributions to the field, particularly in identifying the role of Nox4 (NADPH oxidase-4) in creating scar tissue in the lungs. Dr. Hecker’s goal is to stop Nox4 by developing improved therapies that would help those affected by fibrotic disorders lead longer, healthier lives. Dr. Hecker has received numerous awards and honors for her work, including grants from the National Institutes of Health (NIH) and the U.S. Departments of Defense and Veterans Affairs. She was also named a Distinguished Investigator by the Georgia Research Alliance (GRA) upon joining Emory. In addition, Dr. Hecker has founded two start-up companies related to her research. She has published extensively in peer-reviewed journals and continues to lead innovative research projects aimed at advancing our understanding of pulmonary fibrosis and developing more effective treatments for patients suffering from this condition.

Deborah Bruner, SVP Research; Rasheeta Chandler, inventor; Shweta Ghai, case manager; Robert Nobles, VP Research Admin
Inclusivity, Diversity, and Equity Award (IDEAward): Low English Proficiency Nurse Communication Tool; Christina Calamaro, PhD; Michael Fundora, MD; Morgan Greenleaf; Santiago Arconada Alvarez; Wilbur Lam, MD, PhD
Although the United States continues to become more diverse, English remains the primary language spoken. For patients with limited language proficiency (LLP), navigating a healthcare system that is predominantly English-speaking can be overwhelming. Today, over 8% of the U.S. population identifies as LLP. A language barrier leads to a greater potential for medical errors, therefore a solution to increase the quality and equity of healthcare for LLP patients is urgently needed. Limited language proficiency patients report lower satisfaction overall with health encounters, and they are less likely to understand medical diagnoses and treatment plans. The inventors propose a novel mobile application that enables the clinical team to communicate using common phrases in the patient’s language, images, as well as real-time translations. The app, designed with a pediatric setting in mind, encourages rapid and accurate communication with families by eliminating the wait for an interpreter to be available. Drs. Calamaro and Fundora developed the app in partnership with the Emory AppHatchery. In the future,the team hopes to expand the app’s use to adult patients.

David Mudd, case manager; Robert Nobles, VP Research Admin; Ýmir Vigfússon, inventor; Deborah Bruner, SVP Research
Innovation of the Year: KeyStrike: Securing Communications from an Untrusted Computer; Ýmir Vigfússon, PhD
The last 30 years of computer development and cybersecurity have had one constant: it’s easy to hack a computer. The most common mode of attacks involves compromising the computer of an employee in a number of ways, including phishing campaigns or infected attachments. The level of sophistication of modern attack tools makes detecting whether a computer has been hacked incredibly difficult. Modern cybersecurity is unable to detect if an end-user is physically sitting at an authorized device, or if it has been compromised by a hacker seeking to gain further access into a remote server. To help address this issue, the inventor presents “KeyStrike”, a small USB device that connects to the USB port of a computer. When an end-user enters a key on their keyboard, KeyStrike will forward the keystroke with additional keystrokes. If the server receives a keystroke from the user without the corresponding additional characters, it can be known immediately that the computer may be compromised. Without access to the secret key, the hack would be detected with overwhelming probability.

Robert Nobles, VP Research Admin; Christina Gavegnano, inventor; Deborah Bruner, SVP Research; Laura Fritts, case manager
Deal of the Year: License with Eli Lilly & Company – Specific JAK Inhibitors as Potential Antiviral Agents; Raymond Schinazi, PhD; Christina Gavegnano, PhD
The COVID-19 pandemic has left a devastating impact throughout the world, putting pressure on hospitals and healthcare systems everywhere. At many times, hospitals found themselves overwhelmed with severely ill COVID-19 patients in need of care. The inventors of “Specific JAK Inhibitors as Potential Antiviral Agents” originally intended for their therapeutic to treat HIV with reduced likelihood of resistance and an enhanced safety profile. However, through more research, it was discovered that baricitinib could be used to treat COVID-19 in individuals who require supplemental oxygen or a ventilator. On November 19, 2020, baricitinib was given Emergency Use Authorization from the U.S. Food and Drug Administration. In 2021, Emory University entered into a non-exclusive license agreement with Eli Lilly & Company to treat hospitalized COVID-19 patients with baricitinib.

Robert Nobles, VP Research Admin; John Roback, inventor; Deborah Bruner, SVP Research; Catherine Murari-Kanti, case manager; Edmund Waller, inventor
Start-Up of the Year: Cambium Medical Technologies – Preparation and Usage of Heparin- Conjugated Fibrinogen Depleted Pooled Human Platelet Lysate for Regenerative Medicine; Edmund Waller, MD, PhD; John Roback, MD, PhD
Cambium Medical Technologies was founded in 2013 by four professionals from Emory University. They are focused on developing and commercializing regenerative therapeutics created from novel processed human platelets. Since the licensing of Emory technology “Preparation and Usage of Heparin- Conjugated Fibrinogen Depleted Pooled Human Platelet Lysate for Regenerative Medicine” in 2014, Cambium Medical Technologies has successfully completed a Phase I/II FDA dry eye clinical study for their product Elate Ocular®. In 2021, Cambium received approval from the FDA to begin two Phase III clinical trials for their dry eye biologic.

Robert Nobles, VP Research Admin; David Perryman, George Painter, inventors, Deborah Bruner, SVP Research; David Guthrie, inventor; Laura Fritts, case manager; Stephen Sencer, general counsel
Significant Event of the Year: FDA Emergency Use Authorization of Molnupiravir; Gregory Bluemling, PhD; George Painter III, PhD; Michael Natchus, PhD; David Guthrie, PhD; David Perryman, JD
Throughout the COVID-19 pandemic, there has been an incredibly large strain across the globe. Molnupiravir, an antiviral therapeutic against certain RNA viruses, was invented at Emory through the Drug Innovation Ventures at Emory (DRIVE). The therapeutic was sub-licensed to Ridgeback Biotherapeutics in 2020. It was found that molnupiravir could treat mild-to-moderate COVID-19 infections in those that are at a high risk for hospitalization or death, with the ultimate goal of keeping COVID-19 patients out of the hospital. On December 23, 2021, the U.S. Food and Drug Administration granted Emergency Use Authorization of molnupiravir for the treatment of COVID-19.

Robert Nobles, VP Research Admin; Wilbur Lam, inventor; Deborah Bruner, SVP Research; David Mudd, case manager
Corporate Partnership of the Year: RADx; Wilbur Lam, MD, PhD; Greg Martin, MD, Julie Sullivan
In 2020, Emory University, along with other partners, received a $31 million federal grant from the National Institutes of Health, with the purpose of rapidly transforming innovative technologies into easily accessible diagnostic tests for COVID-19. Researchers at Emory, Children’s Healthcare of Atlanta, and the Georgia Institute of Technology were selected to lead a national effort in testing validation under the umbrella of the Rapid Acceleration of Diagnostics (RADx) program. Over 100 companies participated with RADx to get their tests validated, de-risked, and deployed to market. RADx has since expanded to meet the needs created by the increasing number of variants and the shortage of available tests available to the public, bringing in a total of $64 million of funding. The efforts of the RADx program have helped facilitate FDA authorization for 41 diagnostic tests and have increased testing capacity in the U.S. by more than one billion. Emory OTT provides ongoing support for RADx by efficiently managing the more than 75 research contracts needed to continue their collaborative work with industry and academic partner organizations.

Robert Nobles, VP Research Admin; Nicki Panoskaltsis, inventor; Deborah Bruner, SVP Research; Raj Guddneppanavar, case manager
EmpowHER Award: πChemo/Immuno Precision Medicine Software APP for Leukemia; Nicki Panoskaltsis, MD, PhD
The Emory community is proud to have some of the most cutting-edge research teams led by women. Female scientists at Emory are responsible for a variety of innovative discoveries in biomedical sciences and technology. Some of their inventions have had profound positive impact on the scientific community and society as a whole. Nicki Panoskaltis, MD, PhD and a team of researchers have developed an algorithm that utilizes patient-, leukemia-, and treatment-specific data from standard clinical chemotherapy and immunotherapy treatments. Her goal in doing such is to dynamically predict disease progression prior, during, and post treatment, as well as optimize treatment outcomes through the personalization of drug selection, dosage, and schedule.

Robert Nobles, VP Research Admin; Sarwish Rafiq, inventor; Deborah Bruner, SVP Research; Sunil Raikar, inventor; Raj Guddneppanavar, case manager
Inclusivity, Diversity, and Equity Award (IDEAward): Interleukin-37 Enhances the Effectiveness of CAR T-Cell Therapy; Curtis Henry, PhD; Sunil Raikar, MD; Sarwish Rafiq, PhD
A diverse team of researchers at Emory have developed IL-37-expressing CAR T-cells to treat B-cell acute lymphoblastic leukemia. These cells not only boost the function of aged T-cells, but also enhance the effectiveness of CAR T-cell therapy. Acute lymphoblastic leukemia (ALL) is a highly aggressive type of leukemia and requires very early treatment. Unfortunately, CAR T-cell therapy leads to unsustainable remissions in a majority of patients, and major toxicity due to cytokine release syndrome often occurs. More efficient therapies are highly important in order to increase CAR T-cell longevity and reduce toxicity. In addition to a diverse lab, Curtis Henry, PhD, also serves on the Inclusion, Diversity, Equity and Advancement (IDEA) committee within the Cancer Biology graduate program. This committee’s goal is to take a proactive stance in the creation of an academic environment that is inclusive, welcoming, and celebrates contributions of brilliant scientists from underrepresented groups.
Innovation of the Year: Serological Test for SARS-CoV-2; Mehul Suthar, PhD, Jens Wrammert, PhD, John Roback, PhD, and Rafi Ahmed, PhD
The impact of the COVID-19 pandemic has been devastating across the globe. It is incredibly important to understand the immune response that provides protective immunity to SARS-CoV-2. The inventors of "Serological Test for SARS-CoV-2" investigated the dynamics of the antibody response to the receptor-binding domain (RBD) of the spike protein and virus neutralization activity in acutely infected individuals. Using this information, they have developed an assay capable of detecting the immune response to the SARS-CoV-2 virus at the receptor-binding domain (RBD) of the target sequences. The inventors have also found that serum collected from COVID-19 patients is capable of neutralizing the virus. The results of their research have implications in testing, therapeutics, and vaccine development as it relates to the virus. Their findings illustrate that the RBD assay is highly specific and sensitive, which is important as COVID-19 serological tests are needed to assess immunity and predict patient outcomes.
Deal of the Year: Phaeno, Inc. – A Method for Full-Length RNA Deep Sequencing with Existing Next-Generation Technology; William Agnew, PhD and Mark Emerick, PhD
In 2020, Emory University entered into a license agreement with Phaeno, Inc. for a method for full-length RNA deep sequencing with existing next-generation technology. It is known that it is not only the DNA sequence that drives gene function and protein synthesis. Each gene has numerous splice variants, and some of these can have profound effects on the functions of the proteins they encode. This technology provides a method for rapidly identifying the parent gene of previously unknown splice variant or RNA product. Access and availability of a fast, affordable whole-transcriptome analysis is key for development of targeted medicine due to tissue-specific differences or mutations in protein expression due to cancer. Dr. Agnew and Dr. Emerick have developed a process that combines existing next-generation sequencing technology with unique molecular identification tags to track and identify RNA products back to their originating gene. In July of 2020, Phaeno was accepted by the EvoNexus incubator program after a very rigorous interview process in which Phaeno was evaluated by industry leaders.
Start-up of the Year: IN8bio – Series A Financing; H. Trent Spencer, PhD
IN8bio (formerly Incysus Therapeutics, Inc.) is a clinical-stage biotechnology company whose mission is to develop new, innovative therapies for the treatment of cancer. Their company utilizes gamma-delta T cells to create autologous, allogeneic, and genetically modified therapies for liquid and solid tumors. IN8bio has received substantial financial backing since licensing the "Drug Resistant Immunotherapy for the Treatment of Cancer" technology from Emory University in 2016. Most recently in 2020, IN8bio raised $25.8M capital in a Series A Funding round.
Significant Event of the Year: Aligos Therapeutics, Inc. – Initial Public Offering; Raymond F. Schinazi, PhD; Franck Amblard, PhD; Leda Bassit, PhD
Aligos Therapeutics, Inc. is a clinical stage biotechnology company focused on developing targeted therapies for hepatologic diseases and viral infections. On October 16, 2020, Aligos became publicly traded on the Nasdaq Global Select Market under the ticker symbol "ALGS." Initially priced at $15, the stock ended 2020 at $26.17 and has an all-time high of $37.51. The company's success as a public company comes after two successful financing rounds as a private company: $100M Series A financing round in 2018; oversubscribed $125M Series B financing round. In 2018, Aligos licensed from Emory a HBV capsid inhibitor technology developed in the Laboratory of Biochemical Pharmacology that currently is being evaluated in Phase 1 trials.
Innovation of the Year: Autonomic Formation of Large-Scale Wireless Mesh Networks; Sergio Gramacho & Avani Wildani, PhD
A wireless mesh network (WMN) is a communications network composed of nodes that provide access to the system (e.g., routers) organized in a mesh of linkages. Mesh nodes cooperate by forwarding data on behalf of each other to achieve wide coverage despite using short-range wireless links. WMNs show great promise for enhancing connectivity over large regions at low cost since fewer wired links are required. Capacity scaling is an issue since increasing the number of nodes does not increase its capacity (the amount of traffic that a network can handle at any given time). Also, the typically distributed routing in WMNs is inflexible, hard to adapt to specific use cases. Emory researchers developed a set of self-organizing, self-healing, and self-optimizing autonomic agents (the SMART class of agents) that take an existing WMN node placement and evolve it into interconnected partitions with improved capacity scaling. Moreover, the solution turns practical applying modern paradigms such as Software-Defined Networks, allowing fully programmable routing, at large-scale WMNs. In multiple simulations with thousands of nodes, SMART showed fast convergence to stable partition configurations, and the agent design is able to overcome connectivity failures among the nodes, as well as additional variations in node placement density and scale.
Innovation of the Year: Software to Derive Brand Insights from Mobile Location Data; Daniel McCarthy, PhD & David Schweidel, PhD
Mobile Location Analytics (MLA) is a type of customer intelligence that tracks smartphone locations that can then be matched to locations of interest such as retailers and event venues. By charting the movement of devices and their owners, retailers can gather data that will help them support decisions such as store location, targeted and outdoor advertising, and staffing. Geolocation data has been used to support marketing actions that are credited with increasing retail foot traffic, increasing the likelihood of purchase, and attracting customers from competitors. Emory inventors have developed a privacy-preserving methodology to infer a brand's customer base size and share of wallet within a category by separately identifying customers' location and time-invariant preferences for both brands and specific stores. By analyzing device-level mobile location data, a brand can improve customer relationship management by providing a more complete view of customer behavior, as well as improve store location decisions and real estate decisions, leading to shorter commute times among other time-dependent factors.
Deal of the Year: Kodikaz Therapeutic Solutions – Circulating Tumor DNA for Targeted Cancer Therapeutics; Leon Bernal-Mizrachi, MD
In 2019, Emory University entered into a license agreement with Kodikaz for circulating tumor DNA for targeted delivery of cancer therapeutics. Circulating tumor DNA (ctDNA) is tumor-derived fragmented DNA in the bloodstream that is not associated with cells. Because ctDNA may reflect the entire tumor genome, it has gained traction for its potential clinical utility. Dr. Mizrachi discovered that certain portions of ctDNA make the ctDNA capable of entering the nucleus where it may integrate into the chromosomes of target cells. ctDNA only penetrates the nuclear membrane of the same tissue from which it originated, making ctDNA cell targeting both selective and tissue specific. In 2019, Emory executed a High Net Worth License (potential to generate more than a million dollars in revenue to Emory during the term of the agreement) for this technology.
Start-up of the Year: Aligos Therapeutics, Inc. – Series B Financing; Franck Amblard, PhD & Leda Bassit, PhD & Raymond Schinazi, PhD
Aligos Therapeutics, Inc., is a preclinical stage biotechnology company focused on the development of targeted therapies for hepatologic diseases and viral infections, including chronic hepatitis B (CHB), nonalcoholic steatohepatitis (NASH) and hepatocellular carcinoma (HCC). Aligos has received significant financial backing since licensing the HBV capsid inhibitors technology from Emory in 2018. Most recently in 2019, Aligos closed an oversubscribed $125M Series B equity financing round. Aligos previously closed a $100M Series A financing round. In 2018, Emory University entered into a license agreement with Aligos Therapeutics, Inc. for HBV capsid inhibitors developed in the Laboratory of Biochemical Pharmacology (LOBP). Research in the LOBP focuses on the development of safe and effective antiviral agents.
Significant Event of the Year: Meissa Vaccines, Inc. - License Agreement & Series A Financing; Martin Moore, PhD & Christina Rostad, MD & Sean Todd
Respiratory syncytial virus (RSV) is a common respiratory virus that usually causes mild, cold-like symptoms, but can be serious for infants and older adults. In children younger than one year of age, RSV is the most common cause of bronchiolitis and pneumonia. In children under five, RSV causes more than 30 million new acute lower respiratory infections each year, resulting in more than three million hospital admissions from serious infections around the world. In 2019, Meissa Vaccines, Inc. closed $30 million in series A financing to advance Meissa's investigational RSV vaccine into clinical trials. Meissa Vaccines is a pharmaceutical start-up company focused on advancement of unmet vaccines against viruses that cause serious human diseases, including RSV. In 2017 and 2019, Meissa Vaccines, Inc. entered into license agreements with Emory University for RSV and human metapneumovirus (hMPV) vaccine technologies developed by Emory University researchers in the Division of Pediatric Infectious Diseases.

Thota Ganesh, inventor; Sarah Wilkening, case manager; Deborah Bruner, SVP for Research; Adam Marcus, inventor
Innovation of the Year: Alexidine and Analogs to Treat Lung Cancer; Adam Marcus, PhD & Thota Ganesh, PhD
Lung cancer is the second most prevalent type of cancer, causing more than 150,000 deaths in the United States per year. One of the main barriers in developing new treatment strategies is the vast diversity between and within cancers. The cells within a tumor can vary from one another in many ways such as cellular morphology, gene expression, metabolism, motility, proliferation, or metastatic potential. Cancer cells have the ability to invade adjacent tissues and stimulate neovascularization, which is the critical mechanism of tumor growth and metastasis. Compounds that inhibit this mechanism of metastasis may serve as a potent therapeutic. Dr. Marcus and colleagues have identified a compound, alexidine dihydrochloride, and two analogs that are potent inhibitors of cancer cell invasion, specifically for lung cancer. These compounds shift cancer cells into glycolysis through pyruvate dehydrogenase inhibition. Further work is being done to test the analogs in multiple cancer disease models for anti-metastatic activity. If successful, these compounds may serve as the first anti-metastatic agent that targets invasive cancer cells.

Deborah Bruner SVP for Research; Habib Samady & Alessandro Veneziani, inventors; Raj Guddneppanavar, case manager
Deal of the Year: Covanos, Inc – Vascular Diagnostics Software; Habib Samady, MD & Alessandro Veneziani, PhD
In 2018, Emory University entered into a license agreement with Covanos, Inc. for vascular diagnostics software. Heart disease is the leading cause of death in the United States in both men and women, with over 600,000 deaths each year or one out of every four deaths. Coronary heart disease is the most common type of heart disease and is responsible for almost 400,000 deaths annually. Fractional flow reserve (FFR) is a technique used in coronary catheterization to measure pressure differences across a coronary artery stenosis to determine the likelihood that the stenosis impedes oxygen delivery to the heart muscle. Based on FFR measurement, physicians identify which specific lesion or lesions (or blockage causing blood flow restriction) are responsible for patient's ischemia and warrant stenting or angioplasty. Problems with existing methods of determining FFR include the inability to obtain accurate vessel geometry and the inability to generate active models for inflow/outflow conditions for the computational domain. Drs. Samady, Veneziani, and their team developed a software system for non-invasive determination of FFR. This system utilizes patient-specific noninvasive imaging, physiological measurements, and computational fluid dynamics to determine the FFR. In 2018, Emory executed a High Net Worth License (potential to generate more than a million dollars in revenue to Emory during the term of the agreement) for this technology.

Cale Lennon, case manager; Chip Epstein, inventor; Deborah Bruner, SVP for Research
Start-up of the Year: Neuronetics, Inc.; Chip Epstein, MD
Dr. Charles Epstein is professor in the Department of Neurology in Emory University's School of Medicine. Dr. Epstein's research includes epilepsy, electroencephalogram (EEG), and transcranial magnetic stimulation (TMS). In 2001, Emory University entered into a license agreement with Neuronetics, Inc. for Dr. Epstein's research in magnetic stimulation, which contributed to the success of NeuroStar® Advanced Therapy System. In 2018, Neuronetics closed its initial public offering and closed the year with more than $50 million in total revenue. Neuronetics also launched its first national television advertising campaign for TMS therapy. Neuronetics, Inc. is a commercial-stage medical device company focused on designing, developing, and marketing products that improve the quality of life for patients who suffer from psychiatric disorders. Neuronetics' NeuroStar® Advanced Therapy System, is the leading TMS treatment for major depressive disorder and is backed by the largest clinical data set of any TMS system.

Deborah Bruner, SVP for Research; David Perryman, Abel De La Rosa, Greg Bluemling, & George Painter, inventors; Laura Fritts, case manager
Significant Event of the Year: Antios Therapeutics - License Agreement & Series A Financing; David Perryman, JD, Abel De La Rosa, PhD, Greg Bluemling, PhD, & George Painter, PhD
Hepatitis B virus (HBV) is an infectious disease that targets the liver and results in an acute or chronic infection. An estimated 257 million people live with HBV infection and over 800,000 deaths result from HBV and related complications, which include cirrhosis and hepatocellular carcinoma. Drs. Bluemling, De La Rosa, and Painter developed ATI-2173, Drug Innovation Ventures at Emory (DRIVE)'s leading candidate for the treatment of HBV. DRIVE is a not-for-profit LLC that, while wholly owned by Emory University, functions with the independence of a biotechnology company. DRIVE's research focuses on the discovery and development of nucleoside/nucleotide analogues against infectious diseases like influenza, respiratory syncytial virus infection, chikungunya, viral encephalitis, Ebola, SARS/MERS, and dengue. DRIVE sublicensed its rights to these compounds for the treatment of HBV to Antios Therapeutics. Antios Therapeutics is a biopharmaceutical company devoted to developing innovative therapies for viral diseases. Antios Therapeutics raised $25 million in oversubscribed series A financing to pursue the HBV therapeutic.

David Wynes, VP of Research Administration; Hee Cheol Cho, inventor; Raj Guddneppanavar, case manager
Innovation of the Year: Messenger RNA-Based Biopacemaker; Hee Cheol Cho, PhD
An arrhythmia is a problem with irregular heartbeat. It may feel like the heart skipped a beat, added a beat, is "fluttering," or is beating too fast (tachycardia) or too slow (bradycardia). This is a result of any change from the normal sequence of the electrical impulses in the heart that originate from a tiny region in the heart, which is the pacemaker that we are born with. Millions of people experience arrhythmias due to coronary heart diseases, aging or congenital defects. When the heart beats too slowly, the only medical intervention is implantation of an electronic cardiac pacemaker. The hardware consists of electrical wires that are fixed to the heart muscle and an electronic generator implanted under the chest skin, delivering electrical currents to stimulate the heart to beat. Although they work well, the devices can get infected, cannot adjust the pacing rate on-demand, need battery changes, and are too big for pediatric patients. Dr. Cho and his colleagues have envisioned hardware-free "biological pacemakers" that mimic the natural pacemaker in the heart and solve all the problems associated with device pacing. They have developed a gene therapy, successfully converting ordinary heart muscle into a biological pacemaker in vivo, to provide a safety profile for clinical translation. Dr. Cho teamed up with Dr. Philip Santangelo at Georgia Institute of Technology to deliver the gene as a messenger RNA which sidesteps the problems associated with viral gene therapy vectors. They envision that, for the first clinical trial, the biological pacemakers will serve as adjuncts to electronic devices for patients with temporary pacing needs. If successful, the initial trial will open the door to a global pacemaker industry of $5B/year and growing.

David Wynes, VP of Research Administration; Martin Moore, inventor; Cliff Michaels, case manager
Deal of the Year: Meissa Vaccines Inc. – RSV Vaccine; Martin Moore, PhD
In 2017, Emory University entered into a license agreement with Meissa Vaccines for vaccines for respiratory syncytial virus (RSV). RSV, is a common respiratory virus that usually causes mild, cold-like symptoms with most adults recovering in a week or two. RSV however can be serious, even deadly, especially for infants and older adults with compromised immune systems. Each year in the United States, more than 57,000 children younger than 5 years old are hospitalized due to RSV infection. Additionally, about 177,000 older adults are hospitalized annually in the U.S. with an RSV infection, resulting in approximately 14,000 deaths. Dr. Moore and his colleagues developed a live attenuated RSV vaccine using synthetic biology and reverse genetics to recode non-essential RSV genes and improve stability. This vaccine is the lead product being developed by the licensee. Meissa Vaccines has been awarded a Fast Track SBIR grant by the National Institutes of Health entitled "Development of live attenuated respiratory syncytial virus vaccines with novel thermal stable fusion protein" with a total value of $1.56 million in 2017. It also received a seed investment from FundRx, an innovative healthcare & life sciences venture capital platform based in New York City. This investment is intended to help finance the Company as it moves toward to an investigational new drug (IND) filing for the vaccine.

David Wynes, VP of Research Administration; Monte Eaves, inventor; Kevin Lee, case manager
Start-up of the Year: EMRGE, LLC; Monte Eaves, MD
Felmont F. Eaves III, M.D., F.A.C.S., leads the Emory Aesthetic Center as Medical Director. He is a previous President of the American Society for Aesthetic Plastic Surgery and serves as Director of the American Board of Plastic Surgery, the board that certifies plastic surgeons nationally. During his career, Dr. Eaves has received numerous awards and honors including being recognized as one of the Best Doctors in America continuously for more than a decade. Dr. Eaves is the founder of Emory startup EMRGE, LLC. EMRGE is a medical device company developing simple, cost-effective solutions for wound care, wound closure and scar treatment. The company's three-part platform consists of force-modulating tissue bridges, variable resistance backings and bidirectional linear fixators. Together, these technologies offer a few key needs in wound care: approaches that don't require anesthesia, don't need active removal, minimize risk of infection and reduce scarring. EMRGE plans to market products by the end of 2018.

David Wynes, VP of Research Administration; Wilbur Lam & Erika Tyburski, inventors; Randi Isaacs, case manager
Significant Event of the Year: Sanguina - AnemoCheck 510(k) FDA Approval; Wilbur Lam, M., PhD & Erika Tyburski
Anemia is a condition in which someone's blood has a lower-than-normal amount of red blood cells or hemoglobin. This condition results in the body not receiving enough oxygen rich blood. Currently anemia is monitored via a traditional complete blood count (CBC) test done at the doctor's office. Due to the nature of the blood drawing, this type of testing is both invasive and not appropriate for home testing or self-monitoring purposes. Dr. Lam and Ms. Tyburski, along with colleagues, developed a disposable color-based screening test for anemia, AnemoCheck. This test is used for the determination of hemoglobin level and estimation of hematocrit percentage in whole blood. Each test requires less than half a drop of blood from a finger stick, making it considerably more practical for home use. Blood is collected from the finger stick into a small round device containing a proprietary mix of reagents which react with hemoglobin present in the sample, eliciting a color change. After mixing and waiting for 2 minutes, the resulting color correlates to the patient's hemoglobin level. In 2017, Sanguina, Emory's licensee developing the technology, received 510(k) market clearance from the FDA, and in 2018 plans to launch the product for consumer sale.

Jonathan Lewin, Executive VP Health Affairs; Joshy Jacob, David Holthausen, Song Hee Lee, inventors; Justin Burns, case manager
Innovation of the Year: Frog Skin Peptides as Inhibitors Of Influenza & Zika Virus; Joshy Jacob, PhD, Song Hee Lee, PhD, David Holthausen
Influenza is an infection of the upper respiratory tract caused by the influenza virus. Symptoms can range from muscle ache and runny nose to diarrhea and nausea and may be mild to severe. Worldwide seasonal epidemics can impact 5 – 15% of the population, equating to roughly 3-5 million severe cases and 250, 000- 500,000 deaths worldwide. The emergence of drug resist influenza strains and the occurrence of vaccine mismatch to circulating strain have resulted in current vaccination strategies offering limited protection. Zika is an infectious disease spread primarily by Aedes mosquitoes and results in symptoms similar to those of the flu. Recently, Zika virus has gained notoriety as a cause of Guillain-Barré syndrome and microcephaly, and the virus is being investigated for links to other neurological complications. Currently, no vaccine exists for Zika virus. Emory University researchers have found that peptides secreted through the skin of frogs native to Southern Indian (Hylarana malabarica and Hylarana aurantiaca) have potent virucidal effects against H1 hemagglutinin-bearing human influenza A viruses and Zika virus, respectively. Peptides from Hylarana malabarica effectively limit proliferation of H1N1 influenza strains and peptides from Hylarana aurantiaca are effective against the Asian, African and South American Zika virus strains. These peptides have potential to be developed into potent antiviral therapeutics against both Zika and influenza viruses.

Jonathan Lewin, Executive VP Health Affairs; Dennis Liotta, inventor; Laura Fritts, Director OTT
Deal of the Year: CDK Inhibitors with Carrick Therapeutics; Dennis Liotta, PhD, James Snyder, PhD
Emory University entered into a license agreement with Carrick Therapeutics for highly selective cyclin-dependent kinase (CDK) inhibitors for the treatment of diseases. CDKs are the catalytic subunits of a large family of serine/threonine protein kinases that are integrally involved in a variety of cellular processes. Because of the role of specific CDKs in the regulation of the cell cycle, they have been identified as important targets for the design of drugs with antimitotic, antineurodegenerative, antiviral, and antitumor effects. Emory University researchers identified a series of compounds useful for the selective inhibition of cyclin-dependent kinases, which are being developed by the licensee to treat aggressive forms of cancer in under-served populations. Carrick closed a Series A round of financing, ultimately generating over $90 million in 2016. In 2016, Emory executed a High Net Worth License (potential to generate more than a million dollars in revenue to Emory during the term of the agreement) for this technology.

Jonathan Lewin, Executive VP Health Affairs; Rafael Andino, Clearside Biomedical; Sean Kim, case manager</p><p>
Start-up of the Year: Clearside Biomedical, Inc.; Henry Edelhauser, PhD
Clearside Biomedical, Inc. is a clinical-stage biopharmaceutical company developing first-in-class drug therapies to treat chronic, blinding diseases of the eye. The company's current product candidates focus on diseases affecting the choroid and retina, especially diseases associated with macular edema, and are injected non-surgically into the suprachoroidal space with Clearside's proprietary micro-injector. Clearside's micro-injection platform technology provides targeted delivery of medication specifically to the retina—improving the effectiveness of the drug while reducing side effects that may occur when the drug enters into other parts of the eye. In 2016, Clearside completed its initial public offering. The company raised $50M by offering 7.2 million shares at $7 per share. Clearside also announced the first patient enrolled in Phase 1/2 clinical trial of Zuprata™ in diabetic macular edema and, in December, was added to the Nasdaq Biotechnology Index (NBI). The NBI is designed to track the performance of a set of NASDAQ-listed securities that are classified as either biotechnology or pharmaceutical companies and meet certain eligibility requirements. These requirements include minimum market capitalization, average daily trading volume, and seasoning as a public company.

Jonathan Lewin, Executive VP Health Affairs; Mark Goodman, inventor; Cale Lennon, case manager
Significant Event of the Year: FACBC (Axumin™) FDA Approval; Mark Goodman, PhD
Emory University researchers were investigating a synthetic amino acid labeled with radioactive carbon-11 that had been successfully used to image brain cancer. The problem was that carbon-11 has a short half-life—about 20 minutes—which realistically only allows for one patient study per batch production. Emory University researchers developed FACBC, an analog with a half-life greater than 60 minutes, and studied it in cancer cells and in rodents with grade 4 brain tumor models. FACBC seemed to have the same properties as its carbon-11 labeled predecessor did in humans, as well as a longer half-life from radioactive fluorine-18, which accommodates multidose batch production. The technology was translated to humans and it was found to be superior in glioma and metastatic brain tumor subjects compared to the standard imaging agent at that time. Emory University entered into a license agreement with Nihon-Medi-Physics Co., Ltd. (NMP), which supported Dr. Goodman's research for several years. GE Healthcare (GEHC) sub-licensed the technology from NMP in 2008 and explored applications in several cancers, but eventually focused its efforts on developing an Alzheimer's imaging agent. A few GEHC employees founded Blue Earth Diagnostics in order to bring the imaging agent to market. On May 27, 2016, FACBC, under the brand name Axumin™, gained FDA approval for the detection of recurrent prostate cancer. Axumin™ also received an FDA "orphan drug" designation for the diagnosis of glioma in 2015 and research into several applications of the radiotracer continues at Emory.

David Wynes, VP Research Administration; Khalid Salaita, inventor; Cliff Michaels, case manager; Claire Sterk, Provost
Innovation of the Year: Motion-Based Detection By DNA Machines; Khalid Salaita, PhD
Single Nucleotide Polymorphism (SNP) genotyping is the screening and analysis of genetic variations of SNPs in the genome of various species. SNPs are commonly present in all species including humans. SNP genotyping and analysis technology can analyze thousands of SNPs and has the potential for whole-genome genotyping. DNA-based machines have potential in several applications and industries. Unfortunately, DNA-based walkers are challenging to work with due to their low fidelity and slow rates. Inventors at Emory University have developed a DNA-based machine that can walk by converting chemical energy into controlled motion. This invention shows the first experimental demonstration of self-avoiding motion in DNA walking machines. The motor consists of a DNA-coated spherical particle that hybridizes to a surface modified with complementary RNA. The particle moves upon the addition of RNase H, which selectively hydrolyzes hybridized RNA but not single stranded RNA. This unique cogwheel-like mechanism allows directional motion without the need of a patterned track or external electromagnetic field. Because this new class of DNA-based machines roll rather than walk, they are able to surpass the maximum speed of existing DNA motors by three-orders of magnitude. This technology can serve as a new and powerful tool in SNP genotyping, as well as other applications in diagnostics, drug delivery, and biomaterials.

David Wynes, VP Research Administration; Lisa Matragrano, case manager; Lawrence Wilson, Dennis Liotta, inventors; Claire Sterk, Provost
Deal of the Year: CXCR4 Antagonists with Bristol-Myers Squibb; Dennis Liotta, PhD, Lawrence Wilson, PhD, Michael Natchus, PhD, MBA
Bristol-Myers Squibb, a global BioPharma company in cancer, cardiovascular disease, hepatitis B and hepatitis C, HIV/AIDS, and rheumatoid arthritis, entered into a license agreement with Emory University for small molecule antagonists of CXCR4. CXCR4 expression is low or absent in many healthy tissues, but it was demonstrated to be expressed in over 20 types of cancer, including prostate cancer, ovarian cancer, breast cancer, and melanoma. Emory researchers have developed small molecules which act as antagonists to CXCR4 and may be orally administered. CXCR4 antagonists are known to block adhesion, replication and outgrowth of HIV and can mobilize white blood cells in a dose-dependent manner. Emory researchers are interested in how CXCR4 antagonists may be developed to treat cancers. In 2015, Emory executed a High Net Worth License (potential to generate more than a million dollars in revenue to Emory during the term of the agreement) and research collaboration with Bristol-Myers Squibb for the technology.

David Wynes, VP Research Administration; Mark Goodman, inventor; Cale Lennon, case manager; Kiyoko Takemiya, W. Robert Taylor, inventors; Claire Sterk, Provost
Start-up of the Year: Microbial Medical, Inc.; Mark Goodman, PhD, W. Robert Taylor, MD, PhD, Kiyoko Takemiya, PhD
Microbial Medical develops imaging agents for the non-invasive detection of bacteria in humans. The diagnosis of infections of implanted medical devices is a critical problem. It is estimated that the financial impact of these infections exceeds five billion dollars annually. Although numerous imaging strategies have been developed for diagnosing infections, none of them can detect early stage infections with accuracy or distinguish between infection and inflammation. The inventors developed a technology that employs sugars termed maltodextrins that specifically target the maltodextrin transporter which is uniquely present in all bacteria but not in areas of inflammation. The company's product pipeline includes novel imaging agents and drugs for diagnosing (using common medical scanners) and treating bacterial and fungal infections. In 2015, Emory executed a High Net Worth License (potential to generate more than a million dollars in revenue to Emory during the term of the agreement) with Microbial Medical for the technology.

David Wynes, VP Research Administration; Madhuri Hegde, inventor; Panya Taysavang, case manager; Claire Sterk, Provost
Significant Event of the Year: EGL Genetic Diagnostics, LLC; Madhuri Hegde, PhD
Emory Genetics Lab (EGL) was founded at Emory University in 1970 as a component of the Department of Human Genetics and provides high-complexity molecular, biochemical and cytogenetic testing for rare and common genetic diseases and disorders. EGL serves more than 400 institutional clients, including hospitals and other commercial laboratories across the US and overseas. EGL is renowned for testing rare genetic disorders. The laboratory has comprehensive testing facilities for cytogenetic, biochemical and molecular genetics. As the first academic laboratory to bring Next Generation Sequencing (NGS) technologies to the commercial clinic market, EGL remains a leading player in cutting-edge genetic tests, with "first-to-market" tests comprising more than 80% of its analytical portfolio. As a leading NGS testing laboratory, EGL believes it has the largest menu of NGS panels in the industry, and conducts over 35,000 tests annually for genetic diseases, carrier screening, as well as prenatal testing. Furthermore, EGL is leading the industry in gene panels including cancer testing and exome sequencing, to aid diagnosis, prognosis, management and targeted drug therapy (e.g., personalized medicine), in addition to expanded carrier testing capabilities. In 2015, Eurofins Scientific, the global leader in bio-analytical testing, and one of the world leaders in genomic services, acquired a 75% stake in EGL for approximately US$ 40M in cash. With this acquisition of EGL, Eurofins is expected to further strengthen both its pharmaceutical and genomic service offering and reinforces its development into a leading presence in the specialty clinical testing services for hospitals, clinicians and pharmaceutical companies.

Todd Sherer, Exec Director OTT; Lisa Matragrano, case manager; Kerry Ressler & Raül Andero Galí, inventors
Innovation of the Year: Neurokinin Receptor Blockers for PTSD; Kerry Ressler, MD, PhD, Raül Andero Galí, PhD
Post-traumatic stress disorder (PTSD) occurs in some individuals after witnessing or experiencing extreme traumatic events. Currently those suffering from PTSD are prescribed antidepressants, which have limited success in clinical trials. There are no treatments for specific PTSD symptoms and no effective method for the prevention of PTSD. There are currently only a few clinical and preclinical studies for potential treatments of PTSD. The neurokinin 3 receptor (Nk3R) pathway is involved in the abnormal fear response associated with PTSD. Inhibiting this pathway may serve as a powerful treatment for PTSD and other anxiety disorders. Fear-conditioned mice treated with osanetant, an Nk3R antagonist that failed a Phase II trial for schizophrenia, showed a decrease in fear expression. Although not effective in treating schizophrenia, Nk3R antagonism has been shown to be safe in humans for clinical trials, suggesting that this pharmacological approach could be a safe medication for the treatment of PTSD. Osanetant may also provide a preventative treatment for PTSD wherein osanetant given shortly after a traumatic event could decrease the likelihood of developing PTSD. This treatment has the potential to be applicable in multiple anxiety disorders such as panic disorder, phobias, and PTSD.

Todd Sherer, Exec Director OTT; Cliff Michaels, case manager; Ian Copland, Jacques Galipeau, John Roback & Edmund Waller, inventors
Deal of the Year: Human Platelet Lysate for Regenerative Medicine with Cambium Technologies; Ian Copland, PhD, Jacques Galipeau, MD, Edmund Waller, MD, PhD, John Roback, MD, PhD
Cambium is an early stage company focused on developing therapies from expired blood products. This year Cambium entered into a license agreement with Emory in order to expand their platelet technology portfolio. This license covers technology developed at Emory for a proprietary process of lysing donated platelets and removing fibrinogen while capturing the growth factors. When used as a research reagent, this lysate can stimulate the growth of difficult to culture cell lines. In ex vivo cellular therapies, this lysate can be used to enhance cellular expansion for cell transplant applications. As a direct therapeutic, this lysate can be applied directly to tissues to heal and repair. The company's first clinical application is as a product for treating dry eye syndrome, a condition affecting 25M people in the US alone. In 2014, Emory executed a High Net Worth License (potential to generate more than a million dollars cumulatively over the life of the license) with Cambium for the technology. Also in 2014, Gwowei Technology Co Ltd.'s subsidiary, Amber BioLife Inc., signed a cooperation contract with Cambium Medical Technologies, LLC to manufacture cell culture supplement and develop the dry eye therapy together.

Henry Edelhauser, inventor; Panya Taysavang, Case Manager; Todd Sherer, Exec Director OTT
Start-up of the Year: Clearside Biomedical, Inc.; Henry Edelhauser, PhD
Clearside Biomedical, Inc. is a clinical-stage biopharmaceutical company developing first-in-class drug therapies to treat chronic, blinding diseases of the eye. The company's current product candidates focus on diseases affecting the choroid and retina; especially diseases associated with macular edema, and are injected non-surgically into the suprachoroidal space with Clearside's proprietary micro-injector. Clearside's micro-injection platform technology provides targeted delivery of medication specifically to the retina—improving the effectiveness of the drug while reducing side effects that may occur when the drug enters into other parts of the eye. In a phase 1/2 clinical study of the company's initial product candidate CLS1001, eight patients diagnosed with non-infectious uveitis at three U.S. study centers received a single suprachoroidal injection of preservative-free triamcinolone acetonide injectable suspension using Clearside's proprietary micro-injector. Seven of the eight patients were experiencing macular edema at the time of treatment and all seven achieved clinically meaningful reduction in retinal thickness. In 2014, Clearside raised $16 million in series B financing. Clearside will use proceeds from this financing to fund continued development of the company's proprietary suprachoroidal space (SCS) drug administration, as well as advance two programs into Phase 2 trials.

Todd Sherer, Exec Director OTT; Ian Crocker, inventor; Laura Fritts, Director OTT
Significant Event of the Year: Velocity Medical Solutions' Acquisition by Varian Medical Systems; Ian Crocker, MD
Founded in 2004 at Emory University, Velocity Medical Solutions produces the new imaging software Velocity AITM. This technology can blend cancer images into one high-quality, 3-D like visual that provides a better look at a tumor's actual boundaries. The software does this by matching soft tissue among multiple image sets through image registration, with deformable image registration, which warps images from different angles and with differing contours together, being one of the product's fundamental driving technologies. Velocity AI™ can show a comprehensive view of a patient's diagnostic imaging and treatment history, regardless of where they were treated or what technology was used. The software's interface provides a new and easy-to-use workflow that, together with the technology, will allow physicians to analyze the tumor much faster, cutting the time down from hours to seconds. In addition to efficiency, the technology also provides a more objective way to find the treatment answers that physicians need. Varian Medical Systems agreed to acquire Velocity AITM from Emory University start-up company Velocity Medical Solutions. The Velocity software is already in use at over 200 cancer treatment centers worldwide. Varian expects its global sales, service, and support network will contribute to faster and deeper market penetration for the Velocity product. Varian plans to continue the development of this oncology software platform with the Velocity team in Atlanta, GA.

David Stephens, VP Health Affairs Research; David Wynes, VP Research Admin.; Martin Moore, PhD; Cliff Michaels, case manager
Innovation of the Year: RSV Vaccine Technology, Martin Moore, PhD
RSV is a major cause of lower respiratory tract infections in infants, children, and the elderly. Currently, no RSV vaccine exists. RSV vaccine development has faced challenges including high reversion rate and low immunogenicity. The RSV virion consists of single stranded RNA genetic data, a protein coat, and an envelope that surrounds the protein coat. In addition to the mature viral particle, RSV encodes nonstructural proteins that act within the host cell to suppress an immune response. Most vaccine development has focused on mutating the viral coat, specifically the F and G proteins of the virus. Dr. Moore and colleagues have used an alternative strategy to develop a vaccine that is focused on mutating the genes of the virus that encode nonstructural proteins in order to create a stable, live-attenuated RSV strain. Using a technique called codon de-optimization, a recombinant RSV strain was generated with reduced translational efficiency for the nonstructural genes that results in less expression of the nonstructural proteins. This technique conserves a limited amount of expression of the nonstructural proteins which promotes higher immunogenicity compared to complete inactivation. Additionally through the codon de-optimization technique, the challenge of viral reversion is largely eliminated. The new strain expresses 75% less nonstructural proteins, but retains 100% expression of wild type nucleoproteins and replicates as efficiently as a wild type virus in vitro. In vivo testing is underway as this recombinant RSV strain serves as a promising live, attenuated, vaccine candidate for RSV.

David Stephens, VP Health Affairs Research; James Synder, PhD, inventor; Cale Lennon, case manager; Hyunsuk Shim, PhD, inventor; David Wynes, VP Research Admin.; Mary Galinski, PhD, inventor
Deal of the Year: Sphingolipid Compounds & Anti-Inflammatory Drug with QUE Oncology, Dennis Liotta, PhD, Jim Snyder, PhD, Hyunsuk Shim, PhD, Mary Galinski, PhD
QUE Oncology, headquartered in Atlanta, GA, is focused on the development of novel drug candidates to treat cancer and complications arising from cancer therapy. As a joint venture between Emory University and UniQuest Pty. Limited (Queensland, Australia), QUE Oncology has licensed two technologies from Emory. One technology consists of a set of sphingolipid analogs that target prostate cancer. The second is a novel compound for mitigating hot flashes associated with certain cancer therapies. This clinical stage compound, named Q122, is QUE's most advanced therapeutic development project. Q122 has demonstrated a good safety profile in initial patient dosing studies and is currently being evaluated in a Phase 1b clinical trial. If successful, the compound will fill an unmet need for the treatment of hot flashes in combination with breast cancer therapy. In 2013, Emory executed two High Net Worth Licenses (potential to generate more than a million dollars annually) with QUE for these therapeutic technologies.

David Wynes, VP Research Admin.; David Stephens, VP Health Affairs Research; Stuart Zola, PhD, inventor; Cory Acuff, case manager
Start-up of the Year: Neurotrack, Inc., Stuart Zola, PhD, Eugene Agichtein, PhD, Cecelia Manzanares, & Dmitry Lagun, MS
Neurotrack is an early-stage company that has developed a suite of computer-based tools for recognition memory testing and behavioral assessment. The tools identify those at risk of Alzheimer's disease or mild cognitive impairment well in advance of clinical disease diagnoses. The technology licensed to Neurotrack by Emory consists of software tools and analysis methods that can be used for automatic and accessible administration of cognitive diagnostic tests over the internet. The first generation of the tools relied on hardware that could track eye movements; however the latest generation eliminates this equipment, further improving accessibility and affordability, by relying only on mouse or track pad movements. The redesigned, web-based Visual Paired Comparison test (VPC-W) focuses on a specific type of memory task that depends on the hippocampus, one of the first areas of the brain affected in the course of Alzheimer's disease. Based on a data generated in a 5-year longitudinal clinical study, the technology is able to predict Alzheimer's at least three to four years before clinical symptoms appear with 99% accuracy. This early prognosis should enable patients to receive therapeutic interventions earlier, at a time when the central nervous system is less compromised and potentially more receptive to therapies. The technology was presented at DEMO in Silicon Valley of Fall 2012. In 2013, Neurotrack won the health startup at SXSW and raised $2 million in funding led by Founders Fund and Social+Capital.

Cory Acuff, case manager; David Wynes, VP Research Admin.; Young-sup Yoon, inventor; David Stephens, VP Health Affairs Research
Innovation of the Year: Chemically-Induced Stem Cells, Young-sup Yoon, MD, PhD
Stem cell medicine has been identified as a treatment for a number of conditions including heart disease, diabetes, spinal cord injury, stroke, and cancer; however, obtaining embryonic tissues and isolating relatively rare cell types have limited the large-scale production of populations of pure stem cells. The development of chemically induced pluripotent stem (CiPS) cells provides a patient with a copious, immune-matched supply of pluripotent cells and eliminates ethical issues associated with the production of embryonic stem cells. Current methods used to generate induced pluripotent stem (iPS) cells from somatic cells require overexpression of transcription factors using lentiviral vectors. Unfortunately, this approach involves both viral integration and the use of foreign genes, including genes that could cause cancer. In fact, over 20% of these derived iPS cells develop tumors. In addition, the acquisition of parent cells involves invasive surgical procedures, long-term in vitro manipulation, and possible additional steps to ensure decontamination. This technology describes the use of a unique cocktail of small molecules that can functionally replace exogenous transcription factors to alter gene expression and generate pluripotent cells. The differentiation capacity of the CiPS cells into cell types of all three embryonic germ layers using this technology was similar to embryonic stem cells found in mice. This method will make current and future treatments using stem cells more feasible and affordable.

Cory Acuff, case manager; Dmitry Lagun, Eugene Agichtein, & Elizabeth Buffalo, inventors; David Wynes, VP Research Admin.; Stuart Zola, inventor; David Stephens, VP Health Affairs Research
Deal of the Year: Neurotrack, Inc. License for Web-Based Diagnostic for Alzheimer's Disease, Stuart Zola, PhD; Elizabeth Buffalo, PhD; Cecelia Manzanares; Eugene Agichtein, PhD; Dmitry Lagun, MS
Neurotrack is an early stage company that has developed a suite of computer-based products for recognition memory testing and behavioral assessment tools that allow for identifying those at risk of Alzheimer's disease or mild cognitive impairment in advance of clinical diagnoses. The technology consists of software tools and analysis methods that can be used for automatic and accessible administration of cognitive diagnostic tests over the internet. The first generation of the test relied on an additional piece of hardware that could track eye movements; however the latest generation eliminates this additional equipment, further improving accessibility and affordability, by relying only on mouse or track pad movements. The redesigned, web-based Visual Paired Comparison test (VPC-W) focuses on a specific type of memory task that depends on the hippocampus, one of the first areas of the brain affected in the course of Alzheimer's disease. Based on a 5-year longitudinal study, the test is able to predict Alzheimer's at least three to four years before clinical symptoms appear with 99% accuracy. This early prognosis would enable patients to receive therapeutic interventions earlier, at a time when the central nervous system is less compromised and potentially more receptive to therapies. The technology was presented at DEMO in Silicon Valley of Fall 2012. Earlier in the year, Emory executed a High Net Worth License (potential to generate more than a million dollars annually) with Neurotrack for the technology.

Panya Taysavang, case manager; David Wynes, VP Research Admin.; Samirkumar Patel, GA Tech inventor; David Stephens, VP Health Affairs Research
Start-up of the Year: Clearside Biomedical, Inc., Henry Edelhauser, PhD
Clearside Biomedical has developed an ocular microinjection platform licensed from Emory University and Georgia Tech that allows for non-surgical delivery of drugs to a targeted area of the eye, the suprachoroidal space (SCS) which has only been accessible through surgical techniques in the past. The SCS is the anatomical space between the choroid and sclera and thus suitably located for the delivery of drugs for the retina and choroid. In addition, the SCS can hold up to 200 µL of fluid when injected through the Clearside microinjection platform. The microinjection platform therefore targets drug delivery to the SCS, posterior segment, and retina, and thereby avoids drug exposure and unnecessary side effects to the anterior segment of the eye. In contrast to standard intravitreal injections, this microneedle technology provides a more targeted approach for the treatment of retinal diseases that improves the effectiveness of the drug by confining it to the site of the disease and reduces side effects that can occur when the drug enters other parts of eye. In November 2012 Clearside announced their first successful human dosing in a safety and tolerability study in patients with retinal disease.

Phil Semprevio, case manager; David Wynes, VP Research Admin.; Stephen Warren, inventor; David Stephens, VP Health Affairs Research
Significant Event of the Year: Seaside Therapeutics, Stephen Warren, PhD
Seaside Therapeutics is committed to treating or improving the course of autism, fragile X syndrome, and other neurodevelopmental disorders. While there are treatments used to alleviate some symptoms of neurodevelopmental disorders, there are currently none that address the underlying causes. Seaside's two drug candidates are mGluR5 antagonists and selective gamma-amino butyric acid type B (GABA-B) receptor agonists for the treatment of fragile X syndrome. The mGluR5 antagonists were licensed from Emory. The mGluR5 antagonist is currently in a Phase 2 clinical trial and the GABA-B receptor agonist is currently in Phase 2 and Phase 3 clinical trials. In 2012, Seaside Therapeutics and Roche announced that they had entered into a collaboration agreement to develop disease modifying treatments for both fragile X syndrome and autism spectrum disorders. Seaside will retain rights and continue development of its GABA-B agonists and will license patents covering the mGluR5 antagonists to Roche. Roche will lead the clinical development and commercialization of the mGluR5 antagonists.

David Stephens, VP Health Affairs Research; Murali Padala, inventor; David Wynes, VP Research Admin.; Cory Acuff, case manager
Innovation of the Year: Mitra-Cath Percutaneous Mitral Valve Repair System, Murali Padala, PhD
The Mitra-Cath technology is a novel, catheter-based system for mitral valve repair, which was developed in the Division of Cardiothoracic Surgery at Emory. Mitral regurgitation (MR) often occurs in patients with chronic coronary artery disease or following an acute myocardial infarction. Individuals with MR that is uncorrected develop congestive heart failure, resulting in a significant decrease in their quality of life and often death. Dr. Murali Padala has developed Mitra-Cath, a novel, percutaneous technology to correct MR, which consists of a silicone balloon affixed onto the leaflets of the mitral valve. The device can be implanted into a beating heart via the apex, thus eliminating the need for more risky open heart surgery. The Mitra-Cath technology, which is safe, minimally invasive and adjustable, recently won first place in the SEBIO Bio/Plan Competition in November 2011.

David Stephens, VP Health Affairs Research; Shuming Nie, inventor; David Wynes, VP Research Admin.; Susanne Hollinger, Director OTT
Deal of the Year: Spectropath License for Spectroscopic Intraoperative Tumor Detection System, Shuming Nie, PhD
Spectropath is an optical imaging company that has exclusively licensed novel electronic, optical, chemical, and software technologies developed at Emory University and Georgia Tech that allow physicians to identify selected tissue such as cancer in real time during tumor surgery. Cancer remains a major health issue worldwide, despite continuing progress in therapeutic alternatives. Complete removal of cancer is the single most important predictor of patient survival for most cancers. Presently, the surgeon must rely solely on visual inspection of the surgical field to determine how much tissue to remove. Chemotherapy and radiation treatment are frequently administered as additional prophylactic measures against cancer cells that may not have been removed in surgery. The Spectropath Image-Guided System™ allows the surgeon to actually see cancer cells during surgery. This may provide a level of certainty about the margins of a malignancy that will not only substantially improve patient survival, but may also reduce the need for chemotherapy and radiation treatment, with obvious benefits to quality of life and cost reduction. In 2011 Emory executed a High-Net-Worth License (potential to generate more than a million dollars annually) with Spectropath for the technology.

David Stephens, VP Health Affairs Research; Henry Edelhauser, inventor; David Wynes, VP Research Admin.; Panya Taysavang, case manager</p><p>
Start-up of the Year: Clearside Biomedical, Inc., Henry Edelhauser, PhD
Clearside Biomedical is developing an ocular microinjection platform licensed from Emory University and the Georgia Institute of Technology that allows for targeted non-surgical delivery of drugs to the suprachoroidal space (SCS) of the eye. The SCS is the anatomical space between the choroid and sclera and thus suitably located for delivering drugs to the retina and choroid. It holds up to 200µL of drug and can deliver the drug to areas around the choroid and retina in a sustained release manner. Delivering a drug to the SCS as such, confines it to the diseased tissues of the posterior segment of the eye and retina, and keeps it away from the anterior segment of the eye. The SCS is currently accessible through surgical intravitreal techniques, but Clearside's microneedle technology potentially represents a more focused therapy for treatment of retinal diseases. The microinjection platform targets drug delivery to the suprachoroidal space, posterior segment and retina. The targeted approach improves the effectiveness of the drug while reducing side effects that can occur when the drug enters other parts of the eye. In January 2012 Clearside Biomedical announced that it has secured $4 million from Hatteras Venture Partners in Series A financing of the initial development of the ocular microinjection system for the treatment of macular edema and retinal vein occlusion.

David Stephens, VP Health Affairs Research; Dennis Liotta, inventor; David Wynes, VP Research Admin.; Susanne Hollinger, Director OTT
Significant Event of the Year: Sale of Pharmasset to Gilead, Raymond Schinazi, PhD & Dennis Liotta, PhD
Pharmasset, Inc. is a clinical-stage pharmaceutical company committed to discovering, developing and commercializing novel drugs to treat viral infections. Pharmasset was founded as a start-up company by Dr. Raymond Schinazi and Dr. Dennis Liotta, both faculty members at Emory University. Pharmasset's primary focus has been the development of oral therapeutics for the treatment of infectious diseases, most recently hepatitis C virus (HCV). Current therapeutics for HCV are based on alpha interferon, which is injected once a week and can cause severe flu-like symptoms and other side effects. Pharmasset's push to develop the first all-oral therapy regimen, doing away with the need for interferon, has positioned it at the forefront of HCV treatment. One of Pharmasset's lead drug candidates, PSI-7977 is in phase II clinical testing and could be on the market by 2014. In the fall of 2011, Gilead Sciences announced its intention to purchase Pharmasset for $137 per share, an 81% premium over the company's previous closing price. The deal, which closed on January 17, 2012, was valued at about $11.2 million, primarily based on Pharmasset's oral HCV portfolio.

Cory Acuff, case manager; James Galloway & Niall Galloway, inventors; David Wynes, VP for Research Admin.
Innovation of the Year: Periuretheral Injection Technology for Treating Incontinence, James Galloway, MD & Niall Galloway, MD
Urinary incontinence is a condition that is more often among women and older people. It is estimated that of the 20 million people in the U.S. are affected, between 85% and 90% are women. Bulking agent therapy has been used for over 15 years to treat urinary incontinence. However, it has been reported that many patients need repeat injections within three to five years after the initial injection due to leakage. This flaw is thought to be a result of the technical aspects of the injection that leads to leakage along the needle track or a puncture site rather than a failure of the bulking agent. Naill and James Galloway, MD's at Emory University have created a device that provides the surgeon with a guidance system which improves the technical aspects of the injection by providing a guide for periurethral injection of the bulking agent. This guide minimizes the possibility of needle puncture into the urethral epithelium while creating a longer needle track to discourage leakage. This combination of features provides a standardized technique of injection that produces reproducible outcomes. The device divides the urethra into three distinct regions allowing the injection of three separate bulking agent cushions that will prevent leakage from one region to another. The device is currently being prototyped.

Cory Acuff, case manager; Rafi Ahmed & Jens Wrammert, inventors; David Wynes, VP for Research Admin.
Deal of the Year: Anti-Flu Antibodies Licensing Deal, Jens Wrammert, PhD & Rafi Ahmed, PhD
In a single year, the influenza vaccine only treats two subtypes of influenza A viruses and one influenza B virus. The virus strains selected for the vaccine often change from year to year. Antibodies that show broad cross-reactivity would be useful to develop cross-reactive vaccines that can treat multiple strains of influenza. At Emory University, such antibodies, that show this broad cross-reactivity, were identified. These antibodies would be useful for treating patients with severe influenza or compromised immune systems such as transplant and HIV patients. In addition the identification of these antibodies has provided insights into how to develop broad cross-reactive vaccines for influenza. In 2010, Emory University executed a licensing agreement with a major biotechnology company for these antibodies.

Seth West, GA Tech student; Jorge Jimenez, co-founder; Todd Sherer, Director OTT; Ajit Yoganathan, Vinod Thourani, James Green co-founders; Panya Taysavang, case manager; David Wynes, VP for Research Admin.
Start-up of the Year: Apica Cardiovascular, Inc., Vinod Thourani, MD
Apica Cardiovascular, Inc. (Apica) is a privately held medical device company developing proprietary "Access and Closure" technologies which enable the delivery of large therapeutic devices, such as heart valves for valve replacement and repair, Left Ventricular Assist Devices, and stent grafts for Abdominal and Thoracic Aortic Aneurysm repair. Apica's innovative technology provides access into the beating heart without loss of blood and standardizes the method in which large access sites are managed during the procedure and closed post-procedure via a proprietary sealed conduit and closure system. By eliminating blood loss associated with use of conventional sutures the system improves safety, decreases procedure time, patient costs and technical challenges associated with these minimally invasive procedures. Apica was founded in November, 2009 by Ajit P. Yoganathan, PhD, Jorge Jimenez, PhD, Vinod Thourani, MD, and James L. Greene who are accomplished engineers, entrepreneurs and cardiovascular surgeons with over 80 collective years of medical device development and testing experience. Apica's technology was developed through collaboration between the Georgia Institute of Technology and Emory University, Atlanta, Georgia, U.S.A. Initial grant funding was provided by the Wallace Coulter Foundation and the Georgia Research Alliance's "Venture Lab" program. In 2010, Apica received an exciting €3.75 million investment from Seroba Kernel Life Science, and Triventures, two venture capital firms, allowing the company to continue development and expand.

Cale Lennon, case manager; Pete Lollar, inventor; David Wynes, VP for Research Admin.
Significant Event of the Year: Ipsen Biopharm, John (Pete) Lollar, III, MD
Hemophilia A results from a deficiency of human clotting factor VIII. Approximately 25 percent of severe Hemophilia A patients have a congenital hemophilia characterized by neutralizing antibodies or "inhibitors" to replacement human factor VIII. In the past, these patients were treated with a therapeutic under the name Hyate:C. While this treatment was effective for these patients, its availability was severely and chronically limited due to a difficult manufacturing process. Acquired hemophiliacs or autoantibody patients are not born with hemophilia but develop inhibitors to their own factor VIII. There is no effective therapeutic to treat this condition. Ipsen Biopharm is developing a new therapeutic for patients with both congenital and acquired hemophilia called OBI-1, a recombinant porcine factor VIII (RPFVIII) therapeutic. Because porcine factor VIII has low cross-reactivity with human factor VIII antibodies, OBI-1 is expected to stop bleeding in patients with hemophilia A who have developed inhibitors by engaging the same natural pathway human factor VIII would in noninhibitor patients. In May of 1998, OBI-1 was originally licensed to Octagen. Ipsen was a shareholder of that company. In June of 2008, as part as a strategic decision to begin developing a hematology therapeutic platform, Ipsen took over development of OBI-1 from Octagen. Seven months later Ipsen sublicensed OBI-1 to a hemophilia specialty company Inspiration Biotherapeutics that took over development of OBI-1. Although this technology has had a slow start, due to Ipsen strategically stepping in to take over development of OBI-1, Phase III clinical trials have begun and the first patient was enrolled in December of 2010

Michael Mancini & Aaron Mohs, inventors: Chris Paschall, case manager; Shuming Nie, inventor; David Wynes, VP Research Admin.
Innovation of the Year: Integrated Imaging and Spectroscopy Systems for Image-Guided Surgery (Laser Pen), Shuming Nie, PhD
According to oncologists, successful surgery is the single best predictor of cancer patient survival. However, nearly 40% of cancer patients experience recurrent tumors after their first surgery. The most common operative techniques to locate a tumor in its entirety are inadequate, and cannot always determine the exact location and dimensions of the tumor. Furthermore, reliance on pathology results to confirm tumor detection causes considerable delay in operative and post-operative protocols. Surgeons need better anatomical guidance to excise tumors with high accuracy. To combat this issue, Shuming Nie and his colleagues have developed a novel imaging system that offers precise tumor detection in real-time. The system, comprised of a handheld laser pen and software, is designed to detect the signal from a fluorescent or Raman-active probe introduced into the patient and localized to a tumor or other disease area of interest. The laser pen offers a steady and easy-to-use tool to visualize and excise tumors. Using this system, a surgeon may totally remove a diseased area and verify that the diseased area was successfully and entirely removed. Ultimately, the system should result in a low false-positive rate in tumor identification. Yet another attractive feature of Nie's system is the low cost of manufacture and assembly. Because of the design, engineers can use affordable and readily available parts to construct the system. The device and companion software combine the benefits of real-time spectroscopy, high sensitivity tumor visualization, real-time feedback and instantaneous pathology into a single device. The inventors have been approved to conduct large scale studies in animal models of spontaneous tumors and should initiate that work within the next month.

Cale Lennon, case manager; Jack Arbiser, inventor; David Wynes, VP Research Admin.
Deal of the Year: Alimera Sciences, Inc., Jack Arbiser, MD, PhD
In FY09, Emory and Alimera Sciences, Inc. executed two license agreements covering two separate classes of compounds. The compound classes, broadly known as the fulvenes/fulvalenes and triphenylmethanes, are NADPH (nicotinamide adenine dinucleotide phosphate reduced form) oxidase inhibitors. The licensing of this technology provides Alimera with a platform to develop a novel, therapeutic strategy for the treatment of ocular disorders based on oxidative stress management, specifically the reduction of reactive oxygen species. Potential indications include the dry form of age-related macular degeneration (AMD), particularly the late stage of this condition known as geographic atrophy, as well as diabetic retinopathy. While the dry form of AMD accounts for up to 90 percent of all cases of AMD, no treatment for the dry form of AMD exists. Under the two license agreements, Alimera is granted exclusive rights to commercialize the technologies for opthalmic indications along with an option for non-opthalmic indications.

Cale Lennon, case manager; Stephen Traynelis & Raymond Dingledine, inventors; David Wynes, VP Research Admin
Start-up of the Year: NeurOp, Inc., Raymond Dingledine, PhD & Stephen Traynelis, PhD
NeurOp, Inc., a preclinical stage pharmaceutical company, is developing compounds that target a specific subunit of the N-methyl D-aspartate receptor (NMDAR). Ischemia, depression and pain to are potential markets for NeurOp's NMDAR antagonists. To date, the company has received approximately $2.0M in funding under multiple SBIR awards and has obtained over $500K in funding from angel investors and the State of Georgia. NeurOp has been housed at the EmTech Bio since 2006 and was founded by Emory pharmacologists Raymond Dingledine and Stephen Traynelis and Duke neurologist James McNamara.

Cory Acuff, case manager; Douglas Lowery-North, inventor; David Wynes, VP Research Admin.
Significant Event of the Year: BHR Pharma, LLC, Donald Stein, PhD, Douglas Lowery-North, MD & David Wright, MD
BHR Pharma, LLC (BHR) announced in December 2009 that it filed an Investigational New Drug (IND) application with the US Food and Drug Administration (FDA) for its proprietary BHR-100 intravenous progesterone infusion product. In September 2009, BHR announced it plans to evaluate the safety and effectiveness of BHR-100 as a neuroprotective agent for treating traumatic brain injury (TBI) patients. The Phase III study will include1,200 patients at 100 - 120 sites. Patients will be followed for six months post-injury.
BHR also announced that its BHR-100 product had recently been granted orphan drug status by the FDA Office of Orphan Products Development for early intervention in the treatment of moderate-to-severe closed-head TBI. The FDA only designates orphan-drug status on novel drugs or biologics that treat a rare disease or condition affecting less than 200,000 Americans. The designation offers a number of incentives to the treatment developer. This includes a seven-year period of U.S. marketing exclusivity if the drug receives marketing authorization by the agency. Funding for clinical studies, study design assistance, waiver of FDA user fees and tax credits are additional potential incentives.
BHR holds the exclusive rights to patents licensed from Emory University on the use of progesterone-based drugs for use in treatment of traumatic brain injuries.

Fred Sanfalippo, Exec. VP Health Affairs; Mark Goodman, inventor; Cale Lennon, case manager; David Wynes, VP Research Admin.
Innovation of the Year: Novel PET Imaging Agents, Mark Goodman, PhD
Prostate cancer is one of the most common types of cancer among men. In 2007, 220,000 new cases of prostate cancer were diagnosed and 27,000 deaths resulted from the disease in the United States. While the accurate staging of prostate cancer is essential in order to effectively treat patients, no effective diagnostic imaging agent is available. The diagnostic imaging field of Positron Emission Tomography (PET) is poised for rapid growth with the market in the United States being anticipated to reach $1.0 billion by 2011. Mark Goodman, PhD, in Emory's Department of Radiology has developed unique PET imaging agents to detect and monitor the progression of prostate cancer and other cancers. Rapidly proliferating cells, such as cancerous tumor cells, require high levels of amino acids to divide and grow. The PET imaging agents developed at Emory consist of radiolabeled analogs of amino acids that, when introduced systemically, are more readily taken up by the rapidly dividing cells of a prostate tumor compared to neighboring, non-tumorgenic cells. These compounds have been designed to have high uptake in the prostate with low excretion from the bladder in order to reduce background signal in the groin area.
The imaging agents can also detect prostate cancer that has metastasized to other regions of the body. Goodman and a clinician collaborator are planning to conduct a first-in-man study involving these PET imaging agents at Emory. The study will utilize the exploratory IND (eIND) approval route with the FDA, which is available for microdosing studies in humans where no pharmacological effect from the compound is anticipated (<100 micrograms administered to the subject). The objective of the study will be to gather whole body radiation dosimetry and safety data in healthy volunteers. Distribution of the imaging agent will also be assessed in patients with stages I-IV prostate cancer. GRA, GCC and a private donor have provided $200,000 in seed funding to produce clinical grade compound, perform the required single mammalian species toxicology studies in primates and to perform the first-in-man studies.

Fred Sanfalippo, Exec. VP Health Affairs; Mary Severson, Assoc. Director OTT; Raymond Schinazi, inventor; David Wynes, VP Research Admin.
Deal of the Year: Idenix Pharmaceuticals, Raymond Schinazi, PhD
In July 2008, Massachusetts-based Idenix Pharmaceuticals Inc. settled a long-standing dispute with Emory University and the University of Alabama at Birmingham related to the anti-viral compound, telbivudine, now sold as Tyzeka®/Sebivo® for the treatment of chronic hepatitis B. Upon execution of the settlement agreement, Idenix was obligated to pay the universities $4 million dollars, with additional ongoing royalty and minimum payment obligations related to telbivudine-containing products. Emory's 40% share of payments under this settlement agreement is expected to total at least $6 million dollars by the year 2018.
Chronic hepatitis B is a serious global health problem and many people are not aware that they are infected. WHO reports that approximately 350 million people worldwide have chronic hepatitis B virus (HBV) infection, including, according to the CDC, approximately 1.25 million people in the United States. Approximately 5,000 people in the United States die each year from chronic liver disease related to HBV infection. In the United States, about half of the chronic HBV carriers have been diagnosed, and about 300,000 of these are under a physician's care. It is estimated that only 30,000 of these patients are currently prescribed oral HBV drugs and thus, there exists an unmet medical need in the treatment of HBV that perhaps can be partially fulfilled by telbivudine.

Fred Sanfalippo, Exec. VP Health Affairs; Jennifer Moore, case manager; Charles Epstein, inventor; David Wynes, VP Research Admin.
Start-up of the Year: Neuronetics, Inc., Charles Epstein, MD, PhD
Neuronetics, Inc. (Malvern, PA) was founded in 2003 within The Innovation Factory, an Atlanta-based medical technology incubator, and develops non-invasive therapies for the treatment of severe, chronic psychiatric and neurological disorders. Major depression is a common and serious medical illness affecting more than 13 million Americans, or approximately 6.6 percent of the population, in a given year. It is persistent and can significantly interfere with an individual's thoughts, behavior, mood, and physical health. More than half of the millions being treated for clinical depression, often with complex and sometimes unproven combinations of medications, fail to achieve wellness. Neuronetics' NeuroStar® TMS Therapy provides new hope for patients with major depressive disorder and is based on repetitive transcranial magnetic stimulation (rTMS) technology invented by neurologist Charles M. Epstein, MD, PhD of Emory University. On October 8, 2008, Neuronetics announced that the U.S. Food and Drug Administration (FDA) cleared its NeuroStar® TMS Therapy system for the treatment of depression.
NeuroStar® TMS Therapy is specifically indicated for the treatment of Major Depressive Disorder in adult patients who have failed to achieve satisfactory improvement from one prior antidepressant medication at or above the minimal effective dose and duration in the current episode. The NeuroStar® TMS Therapy system is the first and only TMS Therapy® device cleared by the FDA for the treatment of depression. TMS Therapy® is a non-systemic (does not circulate in the bloodstream throughout the body) and non-invasive (does not involve surgery) form of neuromodulation which stimulates nerve cells in an area of the brain that is linked to depression, by delivering highly focused MRI-strength magnetic pulses. Patients being treated by TMS Therapy® do not require anesthesia or sedation and remain awake and alert. It is a 40-minute outpatient procedure and is typically administered daily for 4-6 weeks.

David Wynes, VP Research Admin.; Dennis Liotta & Raymond Schinazi, inventors; Fred Sanfalippo, Exec. VP Health Affairs; Kevin Lei, Assoc. Director OTT</p><p>
Significant Event of the Year: Pharmasset, Inc., Dennis Liotta, PhD & Raymond Schinazi, PhD
Pharmasset, Inc., founded in 1998 by Emory researchers Dennis Liotta and Raymond Schinazi, is a clinical-stage pharmaceutical company committed to discovering, developing and commercializing novel drugs to treat viral infections. Pharmasset's primary focus is on the development of oral therapeutics for the treatment of hepatitis B virus (HBV), hepatitis C virus (HCV) and human immunodeficiency virus (HIV). The company is currently developing three product candidates. Clevudine, for the treatment of chronic HBV infection, is enrolling Phase 3 clinical trials for registration in North, Central and South America and Europe. Clevudine is already approved for HBV in South Korea and marketed by Bukwang Pharmaceuticals in South Korea under the brand name Levovir. R7128, an oral treatment for chronic HCV infection, is in a 4-week Phase 1 clinical trial in combination with Pegasys® plus Copegus® through a strategic collaboration with Roche. Racivir, which is being developed for the treatment of HIV in combination with other approved HIV drugs, has completed a Phase 2 clinical trial.
In May 2007, the company completed an initial public offering of 5,050,000 shares of common stock at price of $9 a share, resulting in net cash proceeds of $40.7 million. The shares are currently trading above the IPO price at $12.89 (closing price Feb. 19, 2009), in an otherwise difficult market for pharmaceutical companies. Pharmasset reached several milestones in 2008 when it joined the NASDAQ Biotechnology Index on May 19th and the broad-market Russell 3000® Index on June 27th. The NASDAQ Biotechnology Index is the basis for the iShares NASDAQ Biotechnology Index Fund, which seeks investment results that correspond generally to the price and yield performance of the NASDAQ Biotechnology Index. In addition, options based on the NASDAQ Biotechnology Index and the iShares NASDAQ Biotechnology Index Fund trade on various exchanges. Pharmasset's membership in the Russell 3000, which remains in place for one year, means automatic inclusion in the small-cap Russell 2000 Index as well as the appropriate growth and value style indexes. Russell indexes are widely used by investment managers for index funds and as benchmarks for both passive and active investment strategies currently applied to $4.4 trillion in assets.

David Wynes, VP Research Admin.; John Notarantonio, inventor; Panya Taysavang, case manager
Innovation of the Year: G2 Carpool Technology, John Notarantonio
According to the US Census Bureau, nearly 88 percent of workers drive to their jobs, with 77 percent driving alone. John Notarantonio a senior database warehouse developer for Emory University's Technology Services, hopes to reduce that number with a novel "matchmaking" service that makes finding carpooling partners quick and convenient. Emory's 12,000-some employees already have access to the service through Destination Emory, an initiative that encourages alternative commuting options. Employees interested in joining or establishing carpools can navigate to the Destination Emory website, log in, and view a map of their neighborhood with other prospective carpoolers who live and work near them clearly marked. While names aren't supplied, a quick email can be sent through the service and the recipient can respond if they are interested. The model program became the basis for EcoRide™, the only enabling technology specifically designed to address critical environmental and economic issues by stimulating growth in carpooling. EcoRide™ can be used by government agencies, private employers, communities, or individuals as an easy-to-use, easy-to-implement, and cost-effective way to address traffic congestion, limited parking resources, increasing gasoline prices, air quality, and employee health and productivity. Using superior graphics, GIS mapping, and logical data, the system quickly, easily, and securely allows groups to assemble convenient and flexible carpools. Through Emory's Office of Technology Transfer, Notarantonio was paired with Ron Tolliver, a corporate executive with more than 25 years of experience with Fortune 100 and early-stage technology companies, to form G2EcoSolutions and explore the commercial potential of EcoRide™.

David Wynes, VP Research Admin.; Alex Brown (for Rafi Ahmed, Inventor), Cory Acuff, case manager
Deal of the Year: Therapeutic Treatment for Chronic Infections, Rafi Ahmed, PhD
Almost 85% of Hepatitis C (HCV) infections result in a chronic disease state. Compounding this problem is the fact that current HCV therapeutic treatments consisting of pegylated interferon/ribavirin combination therapy are effective in only 50% of individuals having HCV infection. Help, however, is on the way. In 2007, Emory University executed a multi-institutional licensing agreement with a major biotechnology company for the development of therapeutics to treat chronic infection such as HCV and AIDS based on the research discoveries of Rafi Ahmed, PhD and colleagues at the Emory Vaccine Center. Cytokine secreting T cells are the body's natural defense against invading viruses. However, during chronic infection the anti-viral capability of these cells is compromised and they assume a quiescent state known as "T cell exhaustion." Ahmed's team has discovered that CD8 T cells of infected individuals express high levels of an inhibitory receptor known as PD-1, and importantly, virus-infected cells secrete the ligand for PD-1. These discoveries suggest a viral-mediated mechanism for inducing T cell exhaustion, and therefore a potential target for a beneficial intervention. Ahmed and colleagues have identified blocking antibodies to the PD-1 pathway and now, with a biotech partner, will develop therapies to alleviate T cell exhaustion, and help the body defeat chronic infections such as HCV. This exclusive agreement resulted in the largest up-front license payment to Emory over the last five years and an exceptional level of institutional research funding. Notably, the terms of license also include provisions for the Gates Challenge Grant and a global access plan which offer affordable health care opportunities to economically depressed and developing countries. With the basic-science know-how of Ahmed and the commercialization resources of a commercial partner, this deal typifies the reciprocal benefit of institutional-industry collaborations in biotechnology.

David Wynes, VP Research Admin.; Don Hildebrand, GeoVax; Mary Severson, Assoc Director OTT
Start-up of the Year: GeoVax Labs Inc., Harriet Robinson, PhD
Human Immunodeficiency Virus (HIV) remains a viable public health threat in western countries and has reached endemic status in some regions of Africa, a problem compounded by the absence of a viable vaccine. Scientists have struggled unsuccessfully for years to develop a vaccine for the highly mutagenic HIV, a fact illustrated by the large percentage of promising attempts that are abandoned following Phase I clinical trials. However, thanks to the research of Emory scientist Harriet Robinson, HIV vaccine development may have turned a corner. In collaboration with investigators at the National Institutes of Health and Centers for Disease Control and Prevention, Robinson's studies suggested that a DNA vaccine, followed by a MVA booster both encoding multiple HIV proteins could elicit a strong immune response and provide protection against future infections. In 2002, these discoveries were used as the foundation of GeoVax Labs Inc., a new biotechnology company aimed at the development, testing, and commercialization of novel vaccines for infectious diseases including HIV. During a 3 and a 1/2 year pre-clinical trial featuring the company's lead vaccine candidate, a staggering 21 of 23 monkeys were protected from SHIV (hybrid of simian and human HIV). Shortly there after, the GeoVax vaccine was found safe in two Phase I clinical trials and approved for use by the FDA. The vaccine is currently being tested in 3 additional Phase I trials, and in February of 2008, GeoVax announced their product was approved by the HIV Vaccine Trials Network (HVTN) for Phase IIa trials. Significantly, the GeoVax vaccine becomes only the 5th HIV/AIDS vaccine to be selected by HVTN to move forward into Phase II. An excellent 2007 for GeoVax concluded with an award from Georgia Bio for the company's receipt of a $15 million dollar Integrated Preclinical/Clinical AIDS Vaccine Development (IPCAVD) grant from the National Institutes of Health. With this infusion of funding to support their clinical trials program, GeoVax promises to continue their groundbreaking research for years to come.

David Wynes, VP Research Admin.; Dennis Liotta, inventor; Jennifer Moore, case manager
Significant Event of the Year: Triptcor Pharmaceuticals, Dennis Liotta, PhD
Seeking novel therapies for arthritis and other inflammatory diseases Emory scientist Dennis Liotta and colleagues turned to the Triptergium Wilfordii hook F (TWHF) root, a holistic medicine use by the ancient Chinese. TWHF root extracts contain an active ingredient, a small molecule known as triptolide, which research indicates can bind to and block the NF-kB transcription factor, an important mediator of the inflammatory response. Using this knowledge, Liotta and colleagues have developed a series of active triptolide analogs, and subsequently initiated the pre-incorporated start-up company, Triptcor Pharmaceuticals. Founded in 2007, Triptcor was the winner of the 1st annual BIO/plan competition hosted by the regional nonprofit organization Southeast BIO (SEBIO). From an initial applicant pool featuring over 50 innovative technologies from universities and research organizations across the southeast, Triptcor was first selected as one of 10 semi-finalists in June of 2007. Over the next four months a team of Emory representatives, entrepreneurs, and other professionals collaborated to develop a formal business plan. As one of 4 finalists, the Triptcor strategy was presented at the Ninth Annual SEBIO Investor Forum in Pinehurst, N.C., where it was selected by a panel of nine judges as the winner of the $100,000 grand prize. Exceptional research, a well developed business, and exposure generated by this prestigious award combine to generate unprecedented interested from the venture community 2007, and guarantee an exciting 2008 for this budding company.